Enhancing cellulose fiber properties from chromolaena odorata and anana comosus through novel pulping chemical mixtures
Keywords:
Cellulose, Characterization, Lignocellulose, Pineapple leaf, Siam weedAbstract
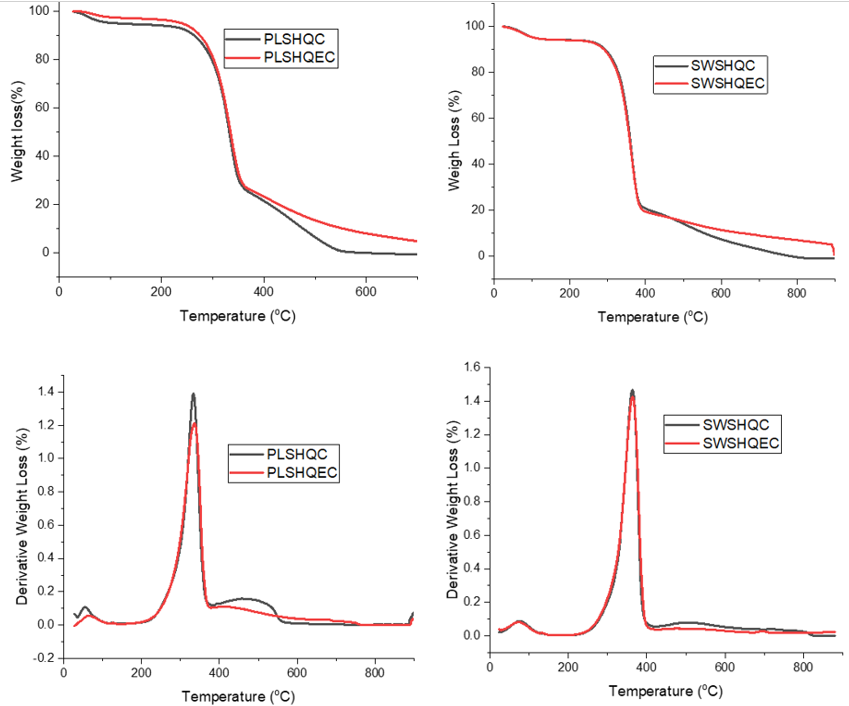
The production of cellulose with exceptional properties has led to the modification of chemical pulping methods and the development of newer chemical pulping methods. This study developed mixtures of sodium hydroxide, ethanol, and anthraquinone as new pulping chemicals to produce alpha-cellulose from pineapple leaves and Saim weed stems. The addition of anthraquinone was noticed to influence the pulp yield positively and the FTIR of all the cellulose has the characteristic cellulose bands. Calculated Total crystallinity index (TCI), (Lateral order index) LOI., and Hydrogen bond intensity (HBI) from the FTIR presented PLSHAEC (68.38 %) and SWSHQEC (67.74 %) having high crystallinity. From the XRD, the 2? by all the materials is attributed to cellulose I diffraction, and the crystallinity index aligned with FTIR determined crystallinity. The determined particle average diameter using ImageJ software showed that PLSHQEC 3.00 had the smallest value, followed by 5.17 for PLSHQC, 5.32 for SWSHQEC, and 6.03 for SWSHQEC. From the thermal analysis, the onset of the degradation of all the cellulose occurred at different temperatures: 247.10? (PLSHQC), 253.38? (PLSHQEC), 240.20? (SWSHQC) and 242.58? (SWSHQEC). The increase in yield, higher crystallinity index, and smaller fiber diameter of the cellulose demonstrated anthraquinone as a better additive to produce quality cellulose that would undergo easy chemical modification.
Published
How to Cite
Issue
Section
Copyright (c) 2024 Olayinka Oluwaseun Oluwasina, Mochamad Zakki Fahmi, Olugbenga Oludayo Oluwasina

This work is licensed under a Creative Commons Attribution 4.0 International License.




