Safranin O dye removal using Senna fistula activated biomass: Kinetic, equilibrium and thermodynamic studies
Keywords:
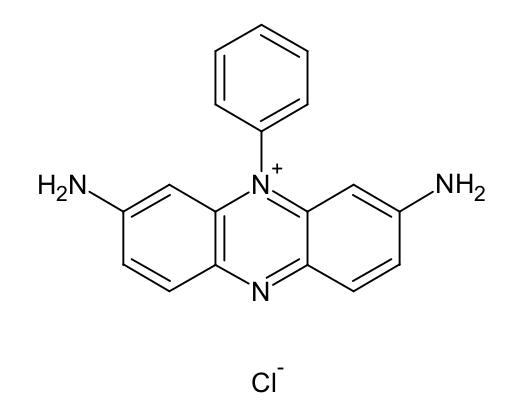
Senna fistula, Safranin O, adsorbent, kinetics, thermodynamicsAbstract
The availability of potable water has decreased in recent times due to the extensive discharge of effluents from some industries. This contaminated water poses a great danger to both human and aquatic life. Senna fistula was activated using phosphoric acid, H3PO4 and its ability to remove Safranin O from aqueous solution was investigated. The characterization of Senna fistula activated carbon was done by Scanning Electron Microscopy and Fourier Transform Infrared Spectroscopy. The impacts of pH, initial dye concentration, contact time, and effect of temperature were investigated. Results showed that the optimum pH for the removal of Safranin O was 4.4. The adsorption capacity increased as the initial dye concentration increased from 30 - 130 mg/L. The dye adsorption equilibrium data were properly fitted to both Freundlich and Langmuir isotherms. The maximum uptake capacity for Safranin O was 22.1 mg/g. The kinetic studies indicated rapid sorption dynamics via a second-order kinetic model. The thermodynamic parameter shows that the sorption of Safranin O on Senna fistula activated carbon was feasible, spontaneous and endothermic. Senna fistula-activated carbon was found to be cheap and efficient adsorbents for the removal of Safranin O dye from aqueous solutions.
Published
How to Cite
Issue
Section
Copyright (c) 2022 C. J. Ajaelu, A. A. Ikotun, E. O. Faboro

This work is licensed under a Creative Commons Attribution 4.0 International License.




